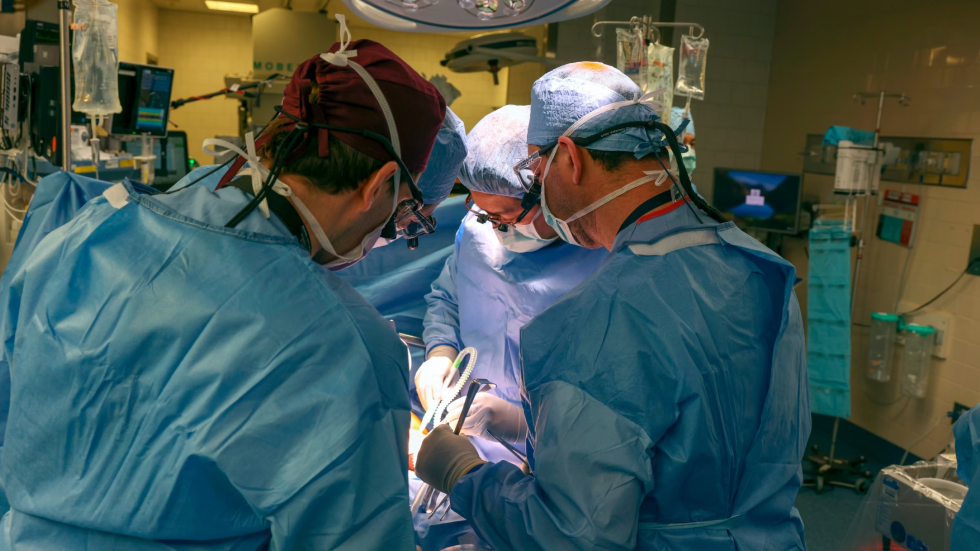
In a groundbreaking medical achievement, doctors at Massachusetts General Hospital (MGH) have successfully completed the world’s first transplantation of a genetically edited pig kidney into a 62-year-old man grappling with end-stage kidney disease (ESKD).
Harvard Medical School (HMS) surgeons orchestrated the four-hour-long procedure on March 16, meticulously transplanting a pig kidney that underwent 69 genomic alterations into the patient, Richard Slayman.
Slayman, who battled type 2 diabetes and hypertension—common triggers for chronic kidney ailments—had previously undergone a human kidney transplant. However, the organ displayed signs of failure after five years, compelling him to resume dialysis due to end-stage kidney failure and frequent hospital visits to manage related complications.
Leading the transformative transplant endeavor, Tatsuo Kawai expressed optimism, stating, “Our hope is that this transplant approach will offer a lifeline to millions of patients worldwide who are suffering from kidney failure.”
The revolutionary process commenced with genetic modifications to render the pig organs more compatible with humans, employing the Nobel Prize-winning gene-editing tool, CRISPR-Cas9. This technology acted as molecular scissors, precisely targeting and altering DNA sequences within the pig kidney.
By excising pig genes responsible for triggering immune responses and integrating select human genes for enhanced compatibility, researchers minimized the risk of rejection. Furthermore, the modifications deactivated viruses inherent in the pig genome, mitigating the threat of infection post-transplantation.
The genetically modified kidney, sourced from eGenesis, a pioneer in xenotransplantation therapy, marked a milestone in medical history. Xenotransplantation, utilizing non-human cells, tissues, or organs to address human medical conditions, found a promising avenue in pigs due to their physiological similarity to humans.
In preparation for the surgery, Slayman received novel monoclonal antibody drugs tailored to suppress immune reactions against pig tissue, ensuring a smoother adaptation post-transplant.
Prior to human application, rigorous testing in animal models validated the safety and efficacy of both the modified organs and immunosuppressant drugs.
Post-surgery, Slayman’s rapid recovery evoked astonishment in the operating room as the newly connected kidney promptly displayed functionality, eliciting applause from the surgical team. Nearly a week later, he continues to progress well, with discharge anticipated soon.
While the immediate success is evident, the long-term durability of the transplant remains uncertain. Kawai conveyed, “We still don’t know how many years this kidney can survive, but based on our preclinical research, we are aiming at years — more than two years.”
Under a single FDA Expanded Access Protocol (EAP), commonly referred to as a compassionate-use protocol, Slayman received approval for the experimental procedure, extending hope to patients facing similar dire circumstances where conventional treatment options are limited or non-existent.